By Rosie Wilson
The consultation, for the Standard titled ‘Aesthetic medicine services – non-surgical medical procedures’, will be held in early 2015.
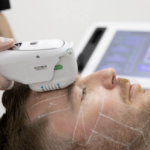
 The European Committee on Standardisation (CEN) have been developing the Standard for four years. It will focus on non-surgical aesthetic treatments, specifically injectables (botulinum toxin and fillers), chemical peels, laser skin resurfacing, skin rejuvenation and hair reduction, amongst others.
The European Committee on Standardisation (CEN) have been developing the Standard for four years. It will focus on non-surgical aesthetic treatments, specifically injectables (botulinum toxin and fillers), chemical peels, laser skin resurfacing, skin rejuvenation and hair reduction, amongst others.
The Public Consultation will be carried out throughout Europe, including the UK, where it will be managed by the British Standards Institution (BSI). Comments will be invited from interested professional bodies as well as from the general public.
This will be the third Public Consultation that has been held by CEN on aesthetic treatments over the past few years. However, where the previous two have invited comments on aesthetic surgical and non-surgical treatments, the latest Public Consultation is more specific. This has arisen from the decision to separate surgical and non-surgical, which was made last year.
The surgical work has now been completed and the resulting European Standard (EN16372) will be published in the next few months.