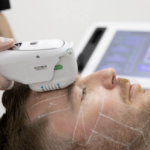
Syneron Medical Ltd, a global market leader in the aesthetic medical device marketplace, announced that it has completed the multi-site clinical study for the UltraShape Contour I System for non-invasive abdominal circumferential reduction.
Syneron Medical Ltd, a global market leader in the aesthetic medical device marketplace, announced that it has completed the multi-site clinical study for the UltraShape Contour I System for non-invasive abdominal circumferential reduction.
The Contour I is designed to use the mechanical effects of ultrasound to disrupt fat cells non-invasively. It achieves this effect without any significant heating of the target or adjacent tissue, minimizing any impact on blood vessels, nerves, and muscle tissue outside the target area. The Contour I also provides an automatic guidance system that adjusts for patient movement for enhanced user convenience and for a high degree of safety.
In the randomized, controlled clinical study of the Contour I, which was performed in 3 clinical sites in the U.S. and one site outside the U.S., a total of 150 subjects were treated and followed-up for up to 4 months. In this multi-site study, an average abdominal circumference reduction of 2.4-3.5 cm was demonstrated. The results successfully met the primary hypothesis for clinically significant circumference reduction. Furthermore, the results demonstrated progressive improvement over time in circumference reduction, beginning after the first treatment session. No serious adverse events related to the device were reported. The treatment was administered without the need for anesthetic and was well tolerated by patients.
The Contour I received the CE mark in 2005 and is marketed in European countries, Canada, Australia, Israel, Mexico, Brazil, Uruguay, Hong Kong, Korea, Russia, Ukraine, Singapore, Taiwan, South Africa and Thailand. The Contour I has been used in 220,000 procedures worldwide, with a positive safety and effectiveness profile.
Read more here: http://www.sacbee.com/2013/10/21/5838757/syneron-medical-completes-and.html#storylink=cpy